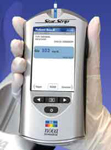
Nova Biomedical announces that its advanced StatStrip™ Point of Care Blood Glucose Monitoring System has received CLIA-waived status from the U.S. Food and Drug Administration (FDA). As a result of StatStrip's waived status, simplicity of use, and accuracy, StatStrip™ can now be operated in point-of-care settings, screening clinics, and non-hospital locations that perform testing under a CLIA certificate of waiver.
Incorporating a patented* new Multi-Wellª strip technology, the StatStrip™ blood glucose monitoring system elevates point-of-care glucose testing to a new level of analytical performance that approaches the quality of central laboratory testing. StatStrip™ measures and corrects hematocrit interference as well as interferences from acetaminophen (Tylenol), uric acid, ascorbic acid (Vitamin C), maltose, galactose, xylose, and lactose. StatStrip™ also eliminates oxygen interference to provide accurate glucose results regardless of the sample's oxygen status.
StatStrip's™ 6-second analysis time, color touch screen operation, and simple operating steps make bedside glucose testing fast and easy for point of care staff. Its small 1.2 microliter sample size results in easy sample acquisition and minimal pain for the patient. Unlike competitive glucose analyzers, StatStrip™ requires no calibration codes thereby eliminating an operator step and preventing a potential input error.
Comprehensive NovaNet software provides management and control of point-of-care testing, allows the StatStrip™ system to be completely customized to the needs of each testing location.
- Product Overview
-
- Educational Resources
-
- Videos
-