Evaluating Memory Loss in Primary Care – A New Approach
Monday, February 07, 2022
By Morgan Ingemanson, PhD
How Do You Evaluate Memory Loss?
When an individual begins to show signs of memory loss, a physician’s greatest challenge is often discovering the underlying cause of symptoms. Behavioral evaluations (including self-report questionnaires such as MoCA and MMSE, effort-based computerized testing, and psychological evaluations) and laboratory tests (such as APOE genotyping and biochemical labs such as blood, urine, and CSF analysis) can be useful in developing a diagnosis in cases of advanced symptom presentation1. But how useful are these tools in assessing cases of early memory loss? Are they capable of detecting dementia early, before disease advancement, so that the physician has the opportunity to implement a successful treatment intervention?
Unfortunately, gold standard assessments often struggle with discovering early stage memory loss. The diagnosis of Alzheimer’s disease (AD) is delayed on average 2-3 years after symptom onset2,3, at a point in which prognosis is poor. Ample clinical research has repeatedly demonstrated that brain changes associated with AD may begin 20 or more years before symptoms appear4. If implemented during this pre-symptomatic stage, lifestyle interventions including diet, exercise, cognitive training and monitoring vascular risk can improve or maintain cognitive functioning and potentially course-correct patients headed down the road to dementia5. Sadly, the tools most often used by physicians to evaluate memory loss are unable to detect this early stage of memory loss.
Moreover, many individuals have dementia-like symptoms without the progressive brain changes of Alzheimer’s disease (AD) or other degenerative brain diseases. Especially in early stages, memory loss related to dementia can be difficult to distinguish from cognitive decline caused by depression, thyroid problems, medication side effects, certain vitamin deficiencies, and even normal healthy aging6. Indeed, up to one in five patients diagnosed with probable AD during their lifetime did not have AD pathology at autopsy7.
How is it possible that current gold standard tools lack the sensitivity and objectivity needed to develop timely and accurate diagnoses for patients experiencing memory loss? It is because memory loss is a brain problem. Symptom screeners and effort-based computerized testing base conclusions off of behavior, not off of how the brain is functioning. Likewise, laboratory tests do not directly assess the main organ of interest. The impairment of memory, language, problem-solving, and other cognitive skills that characterize dementia occurs because neurons in the brain are damaged, destroyed, or dysfunctional. Shouldn’t the highest standard of care for memory loss patients include a direct and objective assessment of the brain itself?
Brain-Based Biomarkers.
Biomarkers are measurable characteristics of a biological function or response that can be objectively measured and evaluated to investigate processes in health and disease. Given that an objectively brain assessment is critical in pinpointing the cause of memory loss, a number of neuroimaging diagnostic biomarkers have been identified for clinical use. These include amyloid deposition as measured by PET scan and cerebral atrophy as measured by MRI8. Unfortunately, in the case of Alzheimer’s disease, these biomarkers begin to present once the disease has already begun to progress (Figure 1). Only once clinical symptoms are evident do various regions of the brain demonstrate atrophy detectible by volumetric MRI4. Moreover, from a practical perspective, these imaging techniques are expensive and/or invasive and therefore remain unavailable to the patients’ first line of contact – the primary care physician.
Electroencephalography, or EEG, involves the measurement of neural oscillations in the brain (i.e., brainwaves), and has long been established as a robust and valuable tool for investigating changes in neural functional. As opposed to techniques that measure brain structure like MRI and CT, EEG evaluates the electrical activity of the brain to reveal how the brain functions. These electrical changes can be measured during a resting state, reflecting spontaneous neural activity, and in “event-related potentials” (ERP), which reflect brain processing speed of environmental stimuli. Due to the un-intrusive, non-invasive, and inexpensive nature of EEG, it is a widely used tool for investigating brain function and neurophysiological health or disease in humans. Additionally, and of particular pertinence, a number of hallmark alterations have been noted in the EEG and ERPs of patients with dementia9.
Up to 20 years prior to the onset of symptoms, there is a window within Alzheimer’s disease progression wherein tau pathology and early amyloid pathology cause impairments in brain function prior to extensive neurodegeneration (Figure 1). Although CSF changes in Aβ and tau are absent at this stage, and functional impairments are not great enough to result in clinical symptoms, clinical research has demonstrated that EEG is capable of detecting biomarkers of impaired brain function. These include spectral “slowing” as measured by quantitative EEG (qEEG), which presents as a relative increase in low frequency brainwaves, and slowed brain processing speed, as indicated by a delay in the P300b component of the ERP waveform9. It is hypothesized that the most effective treatments for AD will be initiated during this early pre-symptomatic stage of the disease, when any damage may be reversed4. Thus, the ability for a primary care physician to collect EEG data and measure these memory loss biomarkers would profoundly improve their ability to make the correct diagnosis and to also provide the prognosis of how to treat the cause.
EEG and ERP for the Primary Care Physician.
Leading research agrees that utilizing traditional clinical evaluation and biochemical labs together with other supportive diagnostic techniques such as functional neuroimaging may be necessary to substantiate the diagnosis of MCI and the subsequent risk of developing AD10 (Figure 2).
With the understanding that the highest standard of care for patients with memory loss includes brain electrophysiology evaluations, comes the need for tools that provide objective memory-related measures that guide and inform physicians in their provision of more targeted therapies. Ideally, such tools would be available in the primary care office, as this is often the first point of care for patients experiencing symptoms of memory loss. However, traditional EEG devices have remained unavailable to most doctors because of expense and complex, time-consuming data interpretation. Historically, only neurologists have had the resources and training to derive clinical significance from EEG data. Moreover, the biomarkers useful in detecting pre-clinical dementia are measured via ERP and quantitative EEG (qEEG) testing, which require sophisticated data processing and comparisons with normative database references values. ERP and qEEG biomarkers provide enhanced objectivity and sensitivity over conventional visual inspection of EEG, yet primary care and specialty physicians alike have not had access to normative database comparisons that permit this type of quantitative analysis.
Advances in data processing and analytics have led to a golden age of electrophysiology assessments. Companies like Evoke Neuroscience (www.evokeneuroscience.com) have created low-cost, easy to use systems designed specifically to suit the needs of primary care physicians. In the case of Evoke Neuroscience’s eVox® System, primary care physicians now have access to these important biomarkers for memory loss to aid their diagnosis. A simple solution of integrated EEG hardware and software allows objective evaluations of brain function that can be performed by office staff. By providing these memory loss biomarkers, the eVox® System supports doctors in objectively assessing patients and may aid in recognizing pre-clinical dementia conditions, identifying the root cause of memory loss, and performing a differential diagnosis.
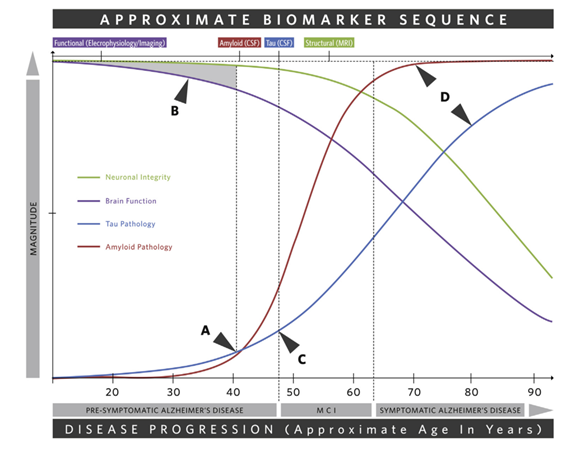
Figure 1. Depiction of the main changes across the progression of Alzheimer’s disease and the sequence of associated biomarkers4. A) Tau and amyloid pathology begin. B) The shaded region indicates a window in which tau and amyloid pathology cause functional impairments that are not great enough to result in clinical symptoms. CSF changes in Aβ and tau are absent but functional biomarkers as measured by EEG and ERP are present. This early pre-symptomatic stage is the time during which the most effective treatments will be initiated and any damage may be reversed. C) Clinical symptoms become apparent. D) Clinical symptoms are progressive and severe. Various brain regions demonstrate atrophy as measured by volumetric MRI.
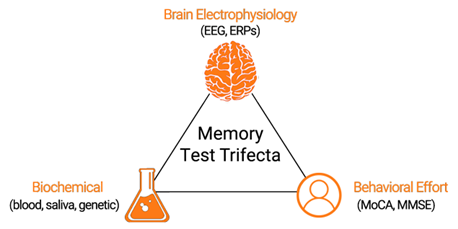
Figure 2. The highest standard of care for patients with memory loss includes assessments of brain electrophysiology, biochemical labs, and effort-based screeners.
References
- National Institute of Health: National Institute on Aging. Diagnosing dementia. Available at: https://www.nia.nih.gov/health/diagnosing-dementia.
- Boise L, et al. Am J Alzheimers Dis. 1999;14:20-26.
- Balasa M, et al. Neurology. 2011;76(20):1720-1725.
- Walsh C, et al. Neurosci Biobehav Rev. 2017;73:340-58.
- Ngandu T, et al. Lancet. 2015; 385(9984):2255-63.
- Alzheimer’s Association. Alzheimer’s Dement. 2018;14(3):367-429
- Beach TG, et al. J Neuropathol Exp Neurol. 2012;71(4):266-273.
- McKhann GM, at al. Alzheimer’s Dement. 2011;7(3):263-9.
- Horvath A, et al. Front Biosci. 2018;23:183-220.
- Ladeira RB, et al. CLINICS. 2009;64(10):967-73.

