 |
BD Veritor System
BD Diagnostics
BD - Diagnostic Systems: BD Veritor System: CLIA-waived For Rapid Detection of Flu A+B.
|

BD Veritor ™ System CLIA-waived for Rapid Detection of Flu A+B meets the New FDA Requirements Our BD Veritor ™ line of immunoassay products, the BD Veritor ™ Plus System and the BD Veritor ™ System give healthcare providers and laboratorians in near patient settings the objective, lab-quality tests results at the point of care within minutes. This assay system eliminates the subjectivity of visual interpretations of tests results and replaces it with an objective digital result. BD Veritor ™ System – Proven Performance vs. Polymerase Chain Reaction (PCR)
Summary of the Performance Data for the BD Veritor System for Rapid Detection of Flu A+B tests compared to PCR for all swabs – all sites 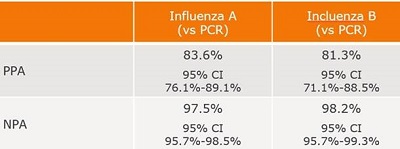 Convenient Workflow 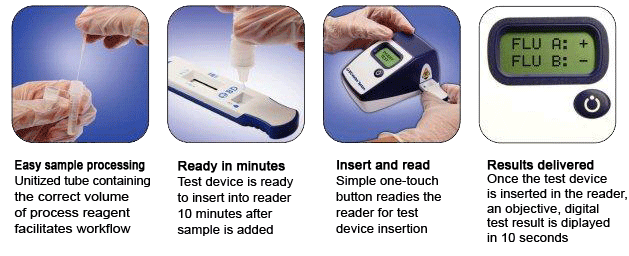 Changing The Way You View Rapid Testing 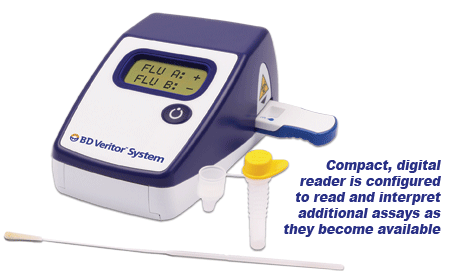
BD Diagnostics
7 Loveton Circle | | Sparks | MD |
BD - Diagnostic Systems: BD Veritor System: CLIA-waived For Rapid Detection of Flu A+B

|
|