 |
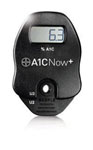
A1CNow+
BAYER HEALTHCARE
A1CNow+ is a portable, easy-to-use system that provides on-the-spot HbA1c result in 5 minutes. This enables the healthcare provider to make immediate treatment decisions and discuss them with patients face-to-face during their visits. The A1CNow+ is portable which enables testing in multiple exam rooms and requires no capital investment. No maintenance, CLIA waived, NGSP certified and reimbursable.
|
|
Purchasing A1CNow+
BAYER HEALTHCARE
510 Oakmead Parkway | | Sunnyvale | CA |
Bayer's A1CNow provides a fast and easy way of obtaining accurate A1C results.

|
|
 |
Hemocue HB 201+
HemoCue America
This is our second generation, improved standard product. Just as earlier versions, this system combines the precision and accuracy of a laboratory measurement with a quick turn around time and ease of use. The pocket size analyzer also stores results and QC results, together with date and time for up to 600 measurements. The stored data can be printed directly via an external printer or downloaded to a PC.
|
|
The HemoCue® Hemoglobin systems provide an opportunity for quick, simple and reliable quantitative hemoglobin results with the same performance as a large hematology analyzer. The dual wavelength analyzers correct for lipemia, leucocytosis and other sources of turbidity. Any blood source (capillary, venous or arterial) can be used. The disposable microcuvette collects the exact amount of blood and mixes the sample with the reagents automatically. The microcuvette is placed into the portable analyzer. Results appear on the display in about a minute. Why to choose a HemoCue® Hemoglobin System
HemoCue® Hb 201+ This is our second generation, improved standard product. Just as earlier versions, this system combines the precision and accuracy of a laboratory measurement with a quick turn around time and ease of use. The pocket size analyzer also stores results and QC results, together with date and time for up to 600 measurements. The stored data can be printed directly via an external printer or downloaded to a PC.
HemoCue America
40 Empire Drive | | Lake Forest | CA |
Since 1982, HemoCue® develops, produces and markets point-of-care medical devices. The unique concept behind each HemoCue® product allows for near-patient testing without sacrificing the precision and accuracy offered by a lab instrument.

|
|
 |
HemoCue Hb 301
HemoCue America
This is a new product, however we don’t have an image. Here’s the product description: The HemoCue® Hb 301 system is optimized for use in primary care and blood donation settings with results in approximately 10 seconds and designed for high temperatures and humidity.
|
|
This is a new product, however we don’t have an image. Here’s the product description: The HemoCue® Hb 301 system is optimized for use in primary care and blood donation settings with results in approximately 10 seconds and designed for high temperatures and humidity. The HemoCue® Hemoglobin systems provide an opportunity for quick, simple and reliable quantitative hemoglobin results with lab quality results. The dual wavelength analyzers correct for lipemia, leucocytosis and other sources of turbidity. Any blood source (capillary, venous or arterial whole blood) can be used. The disposable microcuvette collects the exact amount of blood and mixes the sample with the reagents automatically. The microcuvette is placed into the portable analyzer. Results appear on the display screen in about a minute.
HemoCue America
40 Empire Drive | | Lake Forest | CA |
Since 1982, HemoCue® develops, produces and markets point-of-care medical devices. The unique concept behind each HemoCue® product allows for near-patient testing without sacrificing the precision and accuracy offered by a lab instrument.

|
|
 |
HemoCue Plasma Low/Hb
HemoCue America
HemoCue® Plasma/Low Hb – Photometer for determination of low hemoglobin levels in plasma, serum, or aqueous solutions. There are many occasions when it is necessary to measure low concentrations of hemoglobin in different media. Until now, time-consuming and complicated methods have had to be used or otherwise reliance has had to be made on imprecise visual judgment.
|
|
HemoCue® Plasma/Low Hb – Photometer for determination of low hemoglobin levels in plasma, serum, or aqueous solutions. There are many occasions when it is necessary to measure low concentrations of hemoglobin in different media. Until now, time-consuming and complicated methods have had to be used or otherwise reliance has had to be made on imprecise visual judgment. HemoCue® Plasma/Low Hb Photometer is available and is a quick and simple system, that accurately measures low hemoglobin concentrations in the range of 0—3.00 g/dL (0—30.0 g/L, 0—1.90 mmol/L). Why to choose HemoCue® Plasma/Low Hb System
HemoCue America
40 Empire Drive | | Lake Forest | CA |
Since 1982, HemoCue® develops, produces and markets point-of-care medical devices. The unique concept behind each HemoCue® product allows for near-patient testing without sacrificing the precision and accuracy offered by a lab instrument.

|
|