 |
8500 Pulse Oximeter
NONIN MEDICAL
NONIN's pioneering handheld products offer the best performance on the go. These rugged performers are used every day for anything from a simple spot check to long-term studies. NONIN offers technology you can trust.
|
|||||||||||||||
|
The 8500 Digital Handheld Pulse Oximeter provides a simple universal tool for patient monitoring - from spot-checks to continuous monitoring. handheld products
NONIN MEDICAL
13700 1st Avenue North | | Plymouth | MN |
With over 20 years of experience and dedication to the design and support of noninvasive physiological monitoring devices, Nonin Medical has helped numerous medical professionals worldwide meet clinical and economic objectives. Nonins dedication to technological leadership, precision manufacturing and uncompromised customer support ensures quality products and service our customers can expect.

|
||||||||||||||||
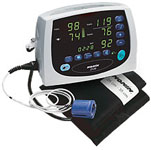 |
Avant® 2120
NONIN MEDICAL
A multi-purpose device offering both noninvasive blood pressure (NIBP) and proven SpO2 monitoring - all in one compact, lightweight and easy-to-use portable unit.
|
|||||||||||||||
|
NONIN MEDICAL
13700 1st Avenue North | | Plymouth | MN |
With over 20 years of experience and dedication to the design and support of noninvasive physiological monitoring devices, Nonin Medical has helped numerous medical professionals worldwide meet clinical and economic objectives. Nonins dedication to technological leadership, precision manufacturing and uncompromised customer support ensures quality products and service our customers can expect.

|
||||||||||||||||
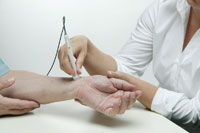 |
SphygmoCor Aortic BP Profile System
ATCOR MEDICAL INC. (USA)
The SphygmoCor Aortic BP Profile system is a non-invasive, doctors office device for obtaining important clinical cardiovascular data. The system does this by analyzing the blood pressure profile where it really matters at the heart. The key to the information the system provides lies in the shape of the aortic pressure wave. This waveform imparts important information about the clinical impact of arterial stiffness on key cardiovascular parameters.
|
|||||||||||||||
|
SphygmoCor Clinical Applications Research Applications The various research areas SphygmoCor has been used in include: Regulatory Status
ATCOR MEDICAL INC. (USA)
One Pierce Place | Suite 295-East | Itasca | IL |
AtCor Medical is the leading developer and marketer of noninvasive technologies and complementary services for the measurement and magagement of central blood pressure and aarterial stiffness.

|
||||||||||||||||
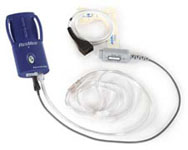 |
ApneaLink
RESMED CORP.
Introducing ApneaLink with oximetry ... your link to increased business and improved patient care.ApneaLink is a simple, cost-effective sleep-screening tool designed to help you increase your business by objectively identifying more patients at risk for obstructive sleep apnea (OSA). ResMed's ApneaLink with oximetry is the premium choice in screening for OSA. The pulse oximeter provides two additional channels of information—pulse and pulse oximetry—in an easy-to-use 'little blue box
|
|||||||||||||||
Features and Benefits
RESMED CORP.
9001 Spectrum Center Blvd. | | San Diego | CA |
Provide global leadership in sleep medicine and non-invasive ventilation based on innovative technology advancing the diagnosis, treatment, and management of sleep-disordered breathing.

|
||||||||||||||||
 |
Sleep Management Kit
RESMED CORP.
Screening your patients for sleep apnea is the first step to treatment. ResMed’s sleep management kit contains tools for physicians in supporting sleep apnea patients, including validated screening questionnaires, product descriptions, script pads, educational posters and brochures, and an informational DVD. These resources are a convenient, effective way to assess your patients for sleep apnea and provide them with the educational materials they need to get started with the appropriate therapy.
|
|||||||||||||||
|
Sleep Management Kit
RESMED CORP.
9001 Spectrum Center Blvd. | | San Diego | CA |
Provide global leadership in sleep medicine and non-invasive ventilation based on innovative technology advancing the diagnosis, treatment, and management of sleep-disordered breathing.

|
||||||||||||||||
 |
Sunlight Omnisense 8000S Bone Densitometry Unit
WALLACH SURGICAL
Mobile bone assessment at multiple skeletal sites is the ideal approach for osteoporosis management. With precise and clinically proven assessment of fracture risk in a mobile format, Omnisense 8000S fits the bill. Omnisense 8000S can be comfortably carried and set up in a new location in under two minutes, making it an excellent choice for users with need for maximum transportability.
|
|||||||||||||||
|
WALLACH SURGICAL
75 Corporate Drive | | Trumbull | CT |
Wallach Surgical Devices is known for leadership and innovation in medical technology. Our commitment to safe, economical medical devices of the highest quality has earned us the trust of the OB/GYN community.

|
||||||||||||||||
 |
ACE Alera® Clinical Chemistry System
ALFA WASSERMANN
Alfa Wassermann Diagnostic Technologies, LLC, specializes in providing clinical chemistry analyzers for the physician's office laboratory. With its expanded test menu of photometric and ISE assays, the ACE Alera Clinical Chemistry System provides the quality of reference laboratory results in a bench top design. The small footprint includes an integrated computer workstation with on-board patient and QC data management, fold down keyboard, LCD monitor and cap piercing of primary tubes.
|
|||||||||||||||
|
The ACE Alera Clinical Chemistry System provides excellent patient care while controlling operating costs.
ALFA WASSERMANN
4 Henderson Drive | | West Caldwell | NJ |
For more than thirty years, Alfa Wassermann, Inc. instruments and products have been used in clinical laboratories all over the world for a wide range of diagnostic applications. These instruments are designed and produced with the aid of the most advanced technology, guaranteeing the accuracy of the laboratories’ analyses.

|
||||||||||||||||
 |
Amplitude T-Series
OTOVATION
Amplitude T-Series wireless audiometer for primary care or specialty physicians, audiologists and on-the-go hearing health professionals. Amplitude T-Series contain all the electronics within the handheld patient response switch, and communicate with a Windows PC by wireless technology. Amplitude T3 performs air and bone conduction testing. Amplitude T4 is the air conduction testing model. Each can be used with a variety of headset transducers. T-Series provide both manual and automated test mod
|
|||||||||||||||
OTOVATION
1001 W. Ninth Ave. | Suite A | King of Prussia | PA |
Otovation was founded in 2003 to design, develop and market advanced diagnostic products for the hearing care market. Since its founding, Otovation has developed and introduced the first portable and wireless systems for both the screening and diagnostic segment of the audiometry market, and we continue to develop advanced technologies and innovative products for improving access to and efficiency of hearing health care delivery.

|
||||||||||||||||
 |
COLA Gives You the Tools to Succeed
COLA
COLA is the premier clinical laboratory education, consultation, and accreditation organization. We are an independent accreditor whose practical, educational standards have a positive and immediate impact. Our services enable clinical laboratories and staff to meet CLIA and other regulatory requirements, act in accordance with Quality Systems, and provide the best possible patient care.
|
|||||||||||||||
|
COLA accredits almost 8,000 medical laboratories and provides the clinical laboratory with a program of education,
consultation, and accreditation. The organization is an independent, non-profit accreditor whose education program and standards enable clinical laboratories
and staff to meet U.S. CLIA and other regulatory requirements. For more information about COLA accreditation services and educational products,
and online educational opportunities, please call 800-981-9883 or email us at info@cola.org.
COLA
9881 Broken Land Parkway | | Columbia | MD |
COLA is a physician-directed organization whose purpose is to promote excellence in laboratory medicine and patient care through a program of voluntary education, consultation, and accreditation.

|
||||||||||||||||
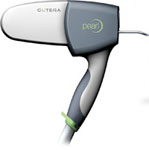 |
Laser
CUTERA, INC.
Pearl is a new, minimally invasive laser that restores the skin’s surface by treating wrinkles, sun damage, uneven texture, and discolorations with fewer treatments and less overall downtime than other technologies. The procedure is fast (only 15 minutes) and uncomplicated (1 or 2 sessions) for a full-face treatment with complete epidermal turnover. Patients feel minimal discomfort and experience only three days of social downtime. Pearl is based on YSGG technology and is the first laser applica
|
|||||||||||||||
CUTERA, INC.
3240 Bayshore Boulevard | | Brisbane | CA |
Cutera’s key messaging is directed to physicians specializing in cosmetic procedures with a focus on four categories of aesthetic solutions and their brands. Strengths are defined through safety, clinical excellence, physician/patient education, customer training and a worldwide service organization. Further, our Solutions are available on upgradeable platforms to protect your investment and avoid obsolesence. Cuteras aesthetic solutions include... Hair Removal full body treatments.

|
||||||||||||||||




