 |
Reveal®G3 Rapid HIV-1
MEDMIRA
Reveal® G3 delivers test results in less than 3 minutes. 3 minute procedure produces instant results. Reveal® G3 Rapid HIV-1 Antibody Test (Reveal G3) is the third generation of MedMira’s Reveal® line of rapid HIV tests. Reveal® G3 is the fastest rapid HIV test available in the US, delivering test results in less than 3 minutes.
|
|
Primary applications for this product include Occupational Exposures, Labor & Delivery and Emergency Departments. The Reveal tests have consistently ranked as a leading choice in rapid HIV tests for US hospitals over the past several years.
MEDMIRA
155 Chain Lake Drive | | Halifax | NS |
We develop and manufacture quality rapid diagnostics that help healthcare providers, public health agencies, and individuals prevent and control the spread of infectious diseases like HIV and Hepatitis, two of the top 10 causes of infectious disease deaths worldwide.

|
|
 |
DAQbilling
ANTEK HEALTHWARE
Antek HealthWare is pleased to offer you DAQbilling, our award-winning medical billing/practice management system. DAQbilling is designed for Private/Group Practices and Billing Services to ease the tedious process of medical billing. DAQ Billing is a comprehensive Medical Billing/Practice Software that will reduce time spent by 30%, increase productivity and reduce spending.
|
|
We invite you to join the over 30,000 physicians enjoying Antek software today. If you thought you would not find a medical billing software system that meets all of your practice's needs, think again. DAQbilling is one of the most comprehensive Medical Billing/Practice Management Software Solutions available on the market today. With such innovative features as the Virtual Lobby, Health Check and Automated Encounters, DAQbilling's capabilities will amaze you. Enjoy the built in efficiency of a complete Practice Management System that covers all bases, including Patient Registration, Appointment Scheduling, Electronic Billing, ERA, Overdue Review, Reporting and Scanning. You will be left wondering why you didn't start looking for a new medical billing software system sooner. Increase efficiency, reduce data entry errors, automate many daily tasks and increase your practice's profitability with DAQbilling today.
ANTEK HEALTHWARE
228 Business Center Drive | | Reisterstown | MD |
Antek HealthWare, LLC is a medical technology solutions provider that delivers advanced clinical laboratory and medical billing software solutions that facilitate and support the practice of medicine.

|
|
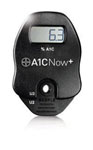 |
A1CNow+
BAYER HEALTHCARE
A1CNow+ is a portable, easy-to-use system that provides on-the-spot HbA1c result in 5 minutes. This enables the healthcare provider to make immediate treatment decisions and discuss them with patients face-to-face during their visits. The A1CNow+ is portable which enables testing in multiple exam rooms and requires no capital investment. No maintenance, CLIA waived, NGSP certified and reimbursable.
|
|
Purchasing A1CNow+
BAYER HEALTHCARE
510 Oakmead Parkway | | Sunnyvale | CA |
Bayer's A1CNow provides a fast and easy way of obtaining accurate A1C results.

|
|
 |
GentleMax
CANDELA
Permanent hair reduction. Leg and facial veins. Even wrinkle reduction and skin tightening. GentleMax gives you the power to do it all with GentleFacial™ approach to skin rejuvenation. Including integrating SmoothPeel™ compact Erbium:YAG laser for superior peels and LightStation 100™ innovative fluorescent pulsed light technology offering a safe and effective skin rejuvenation treatments. See the difference only Candela can make and visit www.gentlemax.com/morepower or to arrange an on-site de
|
|
CANDELA
530 Boston Post Road | | Wayland | MA |
we offer the most comprehensive and technologically sophisticated aesthetic devices in the industry—a remarkably advanced portfolio of aesthetic lasers.

|
|
 |
SmoothPeel®
CANDELA
While chemical peels are among today's most popular aesthetic procedures, they can also be among the most challenging subject to many variables and highly dependent on practitioner interpretation. With SmoothPeel®, Candela's compact Erbium: YAG laser, variables such as differential absorption and timing issues are eliminated. Treatment parameters are determined by controls on the device. Performance is not only more predictable, but repeatable.
|
|
The superior ablative capabilities of the SmoothPeel® 2940nm ER:YAG wavelength are well documented; consistently delivering results that are far beyond microderm abrasion and faster and more controlled than a chemical peel. This precision performance results in greater choice in depth of tissue abrasion. This versatility translates to increased treatment options ranging from very light skin-freshoning treatments with brief downtime, to a more aggressive setting comparable to a glycolic acid peel with 4-7 days downtime.
CANDELA
530 Boston Post Road | | Wayland | MA |
we offer the most comprehensive and technologically sophisticated aesthetic devices in the industry—a remarkably advanced portfolio of aesthetic lasers.

|
|
 |
Vbeam
CANDELA
Perfecta can do it all: from rejuvenation by correcting red and brown skin discoloration, to eliminating virtually all vascular and pigmented lesions. The Perfecta features advanced micro-pulse technology, multiple handpieces for treating various spot sizes, and our patented Dynamic Cooling Device (DCD) cooling system, which all but eliminates patient downtime by preventing purpura, or bruising. And like all Candela products, Perfecta is backed by CandelaÕs unmatched training, marketing and serv
|
|
Applications
CANDELA
530 Boston Post Road | | Wayland | MA |
we offer the most comprehensive and technologically sophisticated aesthetic devices in the industry—a remarkably advanced portfolio of aesthetic lasers.

|
|
 |
HemataSTAT
SEPARATION TECHNOLOGY, INC., PART OF THERMO FISHER
The HemataSTAT II is a self-contained, portable microhematocrit centrifuge that features an easy-to-use, built-in digital tube reader. To use, spin up to six samples for just 60 whisper-quite seconds, position a tube on the built-in reader tray, and move the slider over the tube, making the three sample interfaces. The quantitative hematocrit result is instantly displayed on the LCD panel. Messages guide the operator throughout the testing procedure.
|
|
HemataSTAT®
The Best microhematocrit centrifuge on the market! Can quickly measure hematocrit in any setting. The HemataSTAT® is a microhematocrit system. It provides a quantitative hematocrit. The hematocrit method measures the amount of red blood cells in relation to the amount of plasma. The test requires a small blood sample from a finger stick. HemataSTAT® is also perfect for veterinary use. Features Method
SEPARATION TECHNOLOGY, INC., PART OF THERMO FISHER
1096 Rainer Drive | | Altamonte Springs | FL |
Separation Technology, Inc. providing advanced solutions to the laboratory marketplace since 1988.

|
|
 |
UltraCrit
SEPARATION TECHNOLOGY, INC., PART OF THERMO FISHER
UltraCrit is an FDA cleared device used for donor screening that accurately measures hematocrit with cell counter accuracy. It uses ultrasound technology, which is unlike any competing device. Accuracy is the primary benefit of this technology. Extensive tests show an accuracy of
|
|
Every Hematocrit counts because every donor counts. And that's why there's UltraCrit™.
SEPARATION TECHNOLOGY, INC., PART OF THERMO FISHER
1096 Rainer Drive | | Altamonte Springs | FL |
Separation Technology, Inc. providing advanced solutions to the laboratory marketplace since 1988.

|
|
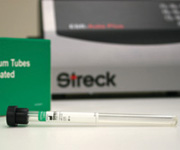 |
ESR-Vacuum Tubes
STRECK LABS
Streck introduces a new safety-coated ESR-Vacuum Tube for Erythrocyte Sedimentation Rate determination on the ESR-Auto Plus™ and ESR-100™ analyzers and the ESR-10 Manual Rack. The new safety-coated tube is the first vacuum ESR blood collection tube that offers the safety characteristics of plastic and the performance and stability of glass. Many laboratories are taking steps to reduce or eliminate the use of glass blood collection tubes. Safety-coated ESR-Vacuum Tubes provide a safer blood colle
|
|
ESR-Vacuum Tubes are evacuated tubes used for the collection of venous blood. ESR-Vacuum Tubes are used to transport and process blood for testing the Erythrocyte Sedimentation Rate (ESR) of whole blood in the clinical laboratory. ESR-Vacuum Tubes are available in 1.2ml and 2.0ml vacuum draw configurations, and are designed specifically for use with the ESR-100 and ESR-Auto Plus automated sedimentation rate analyzers. Blood samples are collected in ESR-Vacuum Tubes containing tri-sodium citrate to avoid coagulation. After the tube is mixed, it is placed in the ESR instrument. Each instrument automatically prints or downloads the ESR result in 30 minutes.
Safety Coated ESR-Vacuum Tubes are designed with an outer Mylar® coating that contains glass and blood specimens in the event of breakage, which reduces the risk of injury and potential exposure to bloodborne pathogens. An independent packaging consultant reports that Safety Coated ESR-Vacuum Tubes are 10 times more impact resistant than glass collection tubes, significantly reducing accidental breakage. ESR-Vacuum Tubes for higher altitude locations are also available. The level of vacuum contained within the high altitude tubes is slightly increased relative to the standard 1.2ml product. The increased vacuum allows venous blood collection draws at high elevations to fill all the way to the desired product fill line. Use of the high altitude tubes at lower elevations (~2500 ft above sea level and below) may result in tubes filling well above the recommended product fill line
STRECK LABS
P.O. Box 45625 | 7002 S. 109th Street | Omaha | NE |
Founded in 1971 and headquartered in Omaha, Nebraska, Streck manufactures hematology, chemistry, and immunology products for the clinical laboratory. Recognized worldwide as a leader in cell stabilization, Streck focuses on the development of products to help meet the fast-paced needs of clinical laboratories.

|
|
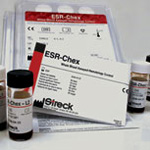 |
ESR-Chex
STRECK LABS
ESR-Chex is manufactured from human red blood cells, and verifies the precision and accuracy of manual and automated methods. ESR-Chex is assayed for the most frequently used sedimentation rate tubes in conjunction with the Classical Westergren, Modified Westergren and Wintrobe methods. Automated and modified procedures do not require removal of sodium citrate, and ESR-Chex is used in the same manner as a patient sample. The control has 95-day open-vial stability at 18°-25°C, and 12-month closed
|
|
ESR-Chex is a two-level hematology control used to monitor erythrocyte sedimentation rates. ESR-Chex is manufactured from human red blood cells, and verifies the precision and accuracy of manual and automated methods. ESR-Chex is assayed for the most frequently used sedimentation rate tubes in conjunction with the Classical Westergren, Modified Westergren and Wintrobe methods. Automated and modified procedures do not require removal of sodium citrate, and ESR-Chex is used in the same manner as a patient sample. The control has 95-day open-vial stability at 18°-25°C, and 12-month closed-vial stability at 2°-10°C.
U.S. Patents 5,895,760; 5,863,799; 5,888,822; 6,017,764; 6,051,433; 6,124,089; 6,159,682; 6,265,148; 6,342,391; 6,331,435; 6,531,321 ESR-Chex Catalog No. 2x9.0ml (Level 1 & 2) 214102 4x9.0ml (Level 1 & 2) 214104 6x9.0ml (Level 1 & 2) 214106 8x9.0ml (Level 1 & 2) 214108 12x9.0ml (Level 1 & 2) 214112 Dispette® 2 Kit: Dispette 2 Tubes (Box of 100) 240300 Dispette Rack, 5-place 240306
STRECK LABS
P.O. Box 45625 | 7002 S. 109th Street | Omaha | NE |
Founded in 1971 and headquartered in Omaha, Nebraska, Streck manufactures hematology, chemistry, and immunology products for the clinical laboratory. Recognized worldwide as a leader in cell stabilization, Streck focuses on the development of products to help meet the fast-paced needs of clinical laboratories.

|
|
