 |
UltraCrit
SEPARATION TECHNOLOGY, INC., PART OF THERMO FISHER
UltraCrit is an FDA cleared device used for donor screening that accurately measures hematocrit with cell counter accuracy. It uses ultrasound technology, which is unlike any competing device. Accuracy is the primary benefit of this technology. Extensive tests show an accuracy of
|
|
Every Hematocrit counts because every donor counts. And that's why there's UltraCrit™.
SEPARATION TECHNOLOGY, INC., PART OF THERMO FISHER
1096 Rainer Drive | | Altamonte Springs | FL |
Separation Technology, Inc. providing advanced solutions to the laboratory marketplace since 1988.

|
|
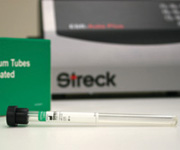 |
ESR-Vacuum Tubes
STRECK LABS
Streck introduces a new safety-coated ESR-Vacuum Tube for Erythrocyte Sedimentation Rate determination on the ESR-Auto Plus™ and ESR-100™ analyzers and the ESR-10 Manual Rack. The new safety-coated tube is the first vacuum ESR blood collection tube that offers the safety characteristics of plastic and the performance and stability of glass. Many laboratories are taking steps to reduce or eliminate the use of glass blood collection tubes. Safety-coated ESR-Vacuum Tubes provide a safer blood colle
|
|
ESR-Vacuum Tubes are evacuated tubes used for the collection of venous blood. ESR-Vacuum Tubes are used to transport and process blood for testing the Erythrocyte Sedimentation Rate (ESR) of whole blood in the clinical laboratory. ESR-Vacuum Tubes are available in 1.2ml and 2.0ml vacuum draw configurations, and are designed specifically for use with the ESR-100 and ESR-Auto Plus automated sedimentation rate analyzers. Blood samples are collected in ESR-Vacuum Tubes containing tri-sodium citrate to avoid coagulation. After the tube is mixed, it is placed in the ESR instrument. Each instrument automatically prints or downloads the ESR result in 30 minutes.
Safety Coated ESR-Vacuum Tubes are designed with an outer Mylar® coating that contains glass and blood specimens in the event of breakage, which reduces the risk of injury and potential exposure to bloodborne pathogens. An independent packaging consultant reports that Safety Coated ESR-Vacuum Tubes are 10 times more impact resistant than glass collection tubes, significantly reducing accidental breakage. ESR-Vacuum Tubes for higher altitude locations are also available. The level of vacuum contained within the high altitude tubes is slightly increased relative to the standard 1.2ml product. The increased vacuum allows venous blood collection draws at high elevations to fill all the way to the desired product fill line. Use of the high altitude tubes at lower elevations (~2500 ft above sea level and below) may result in tubes filling well above the recommended product fill line
STRECK LABS
P.O. Box 45625 | 7002 S. 109th Street | Omaha | NE |
Founded in 1971 and headquartered in Omaha, Nebraska, Streck manufactures hematology, chemistry, and immunology products for the clinical laboratory. Recognized worldwide as a leader in cell stabilization, Streck focuses on the development of products to help meet the fast-paced needs of clinical laboratories.

|
|
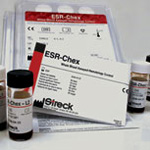 |
ESR-Chex
STRECK LABS
ESR-Chex is manufactured from human red blood cells, and verifies the precision and accuracy of manual and automated methods. ESR-Chex is assayed for the most frequently used sedimentation rate tubes in conjunction with the Classical Westergren, Modified Westergren and Wintrobe methods. Automated and modified procedures do not require removal of sodium citrate, and ESR-Chex is used in the same manner as a patient sample. The control has 95-day open-vial stability at 18°-25°C, and 12-month closed
|
|
ESR-Chex is a two-level hematology control used to monitor erythrocyte sedimentation rates. ESR-Chex is manufactured from human red blood cells, and verifies the precision and accuracy of manual and automated methods. ESR-Chex is assayed for the most frequently used sedimentation rate tubes in conjunction with the Classical Westergren, Modified Westergren and Wintrobe methods. Automated and modified procedures do not require removal of sodium citrate, and ESR-Chex is used in the same manner as a patient sample. The control has 95-day open-vial stability at 18°-25°C, and 12-month closed-vial stability at 2°-10°C.
U.S. Patents 5,895,760; 5,863,799; 5,888,822; 6,017,764; 6,051,433; 6,124,089; 6,159,682; 6,265,148; 6,342,391; 6,331,435; 6,531,321 ESR-Chex Catalog No. 2x9.0ml (Level 1 & 2) 214102 4x9.0ml (Level 1 & 2) 214104 6x9.0ml (Level 1 & 2) 214106 8x9.0ml (Level 1 & 2) 214108 12x9.0ml (Level 1 & 2) 214112 Dispette® 2 Kit: Dispette 2 Tubes (Box of 100) 240300 Dispette Rack, 5-place 240306
STRECK LABS
P.O. Box 45625 | 7002 S. 109th Street | Omaha | NE |
Founded in 1971 and headquartered in Omaha, Nebraska, Streck manufactures hematology, chemistry, and immunology products for the clinical laboratory. Recognized worldwide as a leader in cell stabilization, Streck focuses on the development of products to help meet the fast-paced needs of clinical laboratories.

|
|
 |
ESR-10 Manual Rack
STRECK LABS
The ESR-10 Manual Rack is a 10-position manual Modified Westergren system used for determining the erythrocyte sedimentation rate (ESR) of whole blood in 30 minutes using 1.2ml Streck ESR-Vacuum Tubes. The ESR-10 Manual Rack exhibits >96% correlation to the standard 60-minute manual Modified Westergren method. The rack features a closed blood collection system and is a non-automated (CLIA waived) testing procedure.
|
|
ESR-10 Manual Rack.......................................................Catalog Number
STRECK LABS
P.O. Box 45625 | 7002 S. 109th Street | Omaha | NE |
Founded in 1971 and headquartered in Omaha, Nebraska, Streck manufactures hematology, chemistry, and immunology products for the clinical laboratory. Recognized worldwide as a leader in cell stabilization, Streck focuses on the development of products to help meet the fast-paced needs of clinical laboratories.

|
|
 |
ESR-Auto Plus
STRECK LABS
The ESR-Auto Plus is a 10-position automated ESR analyzer. The instrument accurately and precisely measures the sedimentation rate of erythrocytes in 1.2ml ESR-Vacuum Tubes. Results are measured in millimeters per hour (Modified Westergren Method) and are available in 30 minutes. The instrument features random access, a built-in printer, sample ID capability and a 15-minute prediction mode. The ESR-Auto Plus incorporates a quality control system for monitoring the laboratory's quality control pr
|
|
ESR-Auto Plus Catalog Number
ESR-Auto Plus with testrack: 240357 ESR-657 Mixer: 240316 ESR-Barcode Scanner : 240329 ESR-Vacuum Tubes, 1.2ml (Box of 100): 240360 ESR-Vacuum Tubes, 1.2ml High Altitude (Box of 100): 240213 ESR-Vacuum Tubes, 1.2ml Safety-Coated (Box of 100): 240377 ESR-Vacuum Tubes, 2.0ml (Box of 100): 240320 ESR Printer Paper (5 pack): 240344
STRECK LABS
P.O. Box 45625 | 7002 S. 109th Street | Omaha | NE |
Founded in 1971 and headquartered in Omaha, Nebraska, Streck manufactures hematology, chemistry, and immunology products for the clinical laboratory. Recognized worldwide as a leader in cell stabilization, Streck focuses on the development of products to help meet the fast-paced needs of clinical laboratories.

|
|
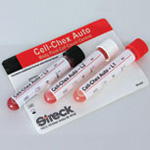 |
Cell-Chex Auto
STRECK LABS
Cell-Chex Auto is a whole blood control for evaluating the accuracy and precision of hematology instruments that measure blood cell counts in patient body fluid samples.
|
|
The assay provides total RBC and WBC values for the Abbott CELL-DYN® 3200, 4000, Sapphire™ and Ruby™; Beckman Coulter® LH 750/LH 755 and LH 780 (levels 2 and 3 only); and Sysmex® XE-2100™, XT-2000i™, and XT-1800i™ instruments. Cell-Chex Auto is a three-level control packaged in a plastic 3.0ml vial with a pierceable cap. Cell-Chex Auto has 75-day closed-vial stability and 30-day open-vial stability when stored at 2¡-10¡C.
STRECK LABS
P.O. Box 45625 | 7002 S. 109th Street | Omaha | NE |
Founded in 1971 and headquartered in Omaha, Nebraska, Streck manufactures hematology, chemistry, and immunology products for the clinical laboratory. Recognized worldwide as a leader in cell stabilization, Streck focuses on the development of products to help meet the fast-paced needs of clinical laboratories.

|
|
 |
HemoCue Hb 201 DM
HemoCue America
The 201 DM systems bring modern Data Management capabilities to the HemoCue:reg; product line. By means of a wide range of settings and definable options, the systems can be made to prompt the operator for identification, lot numbers, quality control and other required information during analysis.
|
|
The 201 DM systems bring modern Data Management capabilities to the HemoCue® product line. By means of a wide range of settings and definable options, the systems can be made to prompt the operator for identification, lot numbers, quality control and other required information during analysis. This data can then be transmitted to clinical information systems. Read more about the Data Management systems. The HemoCue® Hemoglobin systems provide an opportunity for quick, simple and reliable quantitative hemoglobin results with lab quality results. The dual wavelength analyzers correct for lipemia, leucocytosis and other sources of turbidity. Any blood source (capillary, venous or arterial whole blood) can be used. The disposable microcuvette collects the exact amount of blood and mixes the sample with the reagents automatically. The microcuvette is placed into the portable analyzer. Results appear on the display screen in about a minute.
HemoCue America
40 Empire Drive | | Lake Forest | CA |
Since 1982, HemoCue® develops, produces and markets point-of-care medical devices. The unique concept behind each HemoCue® product allows for near-patient testing without sacrificing the precision and accuracy offered by a lab instrument.

|
|
 |
HemoCue Albumin 201 System
HemoCue America
The HemoCue® Albumin systems can be used for the quantitative determination of low levels of albumin in urine for the purpose of screening for, diagnosing, monitoring and supporting the clinical evidence in the treatment of microalbuminuria.
|
|
HemoCue® Albumin 201 System The HemoCue® Albumin systems can be used for the quantitative determination of low levels of albumin in urine for the purpose of screening for, diagnosing, monitoring and supporting the clinical evidence in the treatment of microalbuminuria. The system consists of a analyzer and individually packaged microcuvettes. The reaction in the cuvette is based on a immunoturbidimetric reaction. The turbidity once formed is measured photometrically at 610 nm. With three simple steps a quantitative result is achieved within 90 seconds! Why to choose HemoCue® Albumin 201 System...
HemoCue America
40 Empire Drive | | Lake Forest | CA |
Since 1982, HemoCue® develops, produces and markets point-of-care medical devices. The unique concept behind each HemoCue® product allows for near-patient testing without sacrificing the precision and accuracy offered by a lab instrument.

|
|
 |
HemoCue WBC Systems
HemoCue America
The HemoCue® WBC system is a unique point-of-care testing system for the quantitative determination of white blood cell (WBC) count. Based on the HemoCue® proven and reliable microcuvette technology, lab quality results are obtained from a finger stick in minutes.
|
|
The HemoCue® WBC system is a unique point-of-care testing system for the quantitative determination of white blood cell (WBC) count. Based on the HemoCue® proven and reliable microcuvette technology, lab quality results are obtained from a finger stick in minutes. The HemoCue® WBC system only requires 10 µL of whole blood from a finger-stick or a venous EDTA sample. A hemolyzing agent lyses the red cells in the microcuvette and a staining agent colors the white cells. An image is taken of the stained white cells and the number of cells is then counted by image analysis in the analyzer. Unlike conventional laboratory methods, which measure red and total blood cell counts; the HemoCue® WBC system is a point-of-care device that provides quantitative results of a patient's white blood cell count in about 3 minutes. This will assist the physician in evaluating a patient's condition, while the patient is still in his/her office. Why to choose HemoCue® WBC System...
HemoCue America
40 Empire Drive | | Lake Forest | CA |
Since 1982, HemoCue® develops, produces and markets point-of-care medical devices. The unique concept behind each HemoCue® product allows for near-patient testing without sacrificing the precision and accuracy offered by a lab instrument.

|
|